
UltraScan™ 650 ‒‒ The only ultrasound bone densitometer to replace DXA for measuring BMD
|
Net Time Delay (NTD) Technology UltraScan™ 650 relies on CyberLogic’s patented NTD technology. The NTD is based on measuring transit times of ultrasound signals propagating through the forearm, which allows direct measurement of the amount of bone. The NTD has been validated with computer simulations, in vitro studies, and clinical data, and represents a completely distinct approach to processing of ultrasound data. Conventional bone sonometers evaluate the speed of sound (SOS) and broadband ultrasound attenuation (BUA); these parameters are only weakly correlated with bone mineral density (BMD). In contrast, the high degree of correlation (r = 0.93) of the UltraScan 650 output with BMD is due to the use of the NTD technology. A number of papers and patents related to the NTD technology can be found in the References. Specifically, a summary of the key clinical data can be found in: (A comprehensive paper summarizing the performance of the UltraScan 650 in a clinical trial can be found in our publication by Stein et al. in Ultrasound in Medicine & Biology.) The simulation and in vitro data can be found, respectfully, at: "Ultrasound Simulation in Bone" and "Ultrasonic Assessment of the Radius in vitro".
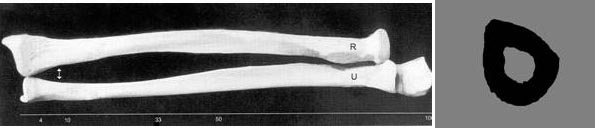
The UltraScan 650 utilizes the 1/3 radius for determination of BMD. The 1/3 radius has been used for over 5 decades by x-ray methods (SPA, DPA, SXA, DXA) and is well-established as a useful anatomical site for bone assessment and osteoporotic fracture risk determination. It is also an extremely convenient site, and generally requires nothing more than rolling up one’s sleeve. The figure shows the two bones of the forearm (radius and ulna) as well as the cross section of the radius. Note that the UltraScan 650 is the only through-transmission ultrasound device approved in the U.S. for measurement at the 1/3 radius, and the only one approved by the FDA for measurement of BMD.
FOREARM POSITIONING TECHNOLGY The UltraScan 650 incorporates patented positioning technology, which makes for both reproducible and accurate measurement of BMD at the 1/3 radius. Whereas conventional ultrasound measurements of the heel lead to great variations in results because of the difficulty in re-positioning, the UltraScan 650 can be respositioned extremely accurately. In addition, standard DXA requires significant technician interaction in terms of processing the data; in contrast, the UltraScan 650 does not require nor does it permit any user interactions with data processing.
Device Description: The UltraScan 650 is an ultrasound device that is designed to non-invasively and quantitatively assess the amount of bone at the 1/3 location of the radius in the forearm of an individual. The UltraScan 650, with a user-supplied laptop, is designed for the measurement of bone mineral density (BMD in g/cm2) of the radius at the 1/3 location. The UltraScan 650 outputs BMDUS which is a measurement of the BMD that would be measured by dual-energy X-ray absorptiometry (DXA) at the same anatomical location, that is, a measurement of BMDDXA, at the 1/3 radius. The UltraScan 650 also outputs the T-score in standard deviations (SD) and z-score in SD as well. The precision of the measurement is 2.1%, when expressed as a coefficient of variation. FDA-approved Indications for Use: UltraScan 650 can be used to determine BMDUS Index in adult men and women and to assess appendicular fracture risk in postmenopausal women. The BMDUS Index is a clinical measure based on ultrasound variables of the forearm which is highly correlated with the value of BMD of the 1/3 radius as provided by DXA, with a standard error of the measurement of 0.041 grams/cm2. BMDUS Index is expressed in grams/cm2 and as a T- and z-score, derived from comparison to a normative x-ray absorptiometry reference database. BMDUS Index has a precision comparable to that of x-ray absorptiometry, which makes it suitable for monitoring bone changes in postmenopausal women. The UltraScan™ 650 is a through-transmission system and is designed to measure the radius at the 1/3rd location (shown as the radius and ulna and the cross-section of the radius in the pictures above), a site that has been measured for decades with x-ray densitometers.
Innovation Summary The UltraScan 650 will expand access to bone assessment and significantly decrease the under-diagnosis of osteoporosis. Clinical studies show the correlation between BMD of a DXA and UltraScan 650 is 0.93. The portability and simplicity in use of the radiation-free ultrasound scanner, combined with its high degree of accuracy and precision in measuring radial BMD, provides a basis by which to expand ultrasonic assessment to the primary care setting. This will in turn provide an opportunity to reduce the incidence of osteoporotic fractures through early and timely therapeutic interventions, and thereby reduce the degree of associated morbidity and mortality.
|
© 2024 CyberLogic, inc. All Rights Reserved